Question #83d74
1 Answer
Electron configurations of transition metals are in general not necessarily intuitive at first glance.
BASIC ELECTRON CONFIGURATIONS
The first-row transition metals are where we first introduce the explicit usage of the
It's where the electron configuration goes
IONIZATION OF TRANSITION METALS (FIRST-ROW)
Most professors will tell you that the
It's good to compare to other sources (trust but verify!), and this PDF from my Inorganic Chemistry textbook says that most transition metals have orbital potential energies higher for the
http://media.pearsoncmg.com/bc/bc_0media_chem/adv_chem/pdf/11054_appB_ts.pdf
Then, it makes sense that the
Furthermore, even if it were really the case that the
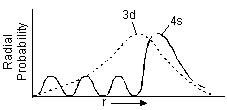
Thus, it is indeed easier to ionize a transition metal's

