Question #79430
↳Redirected from
"Question #79430"
1 Answer
Feb 15, 2014
Polyatomic ions are covalently bonded within the ion, but they form ionic bonds to other ions.
Explanation:
The only difference between a molecule and an ion is the number of valence electrons.
Since molecules are covalently bonded, their polyatomic ions are also covalently bonded.
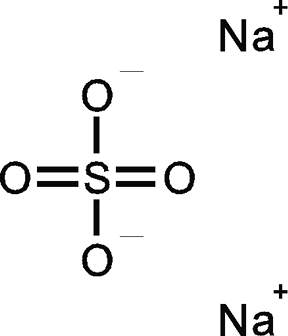
For example, in the Lewis structure of sulfate ion,

Once the sulfate ion,