What are quantum numbers?
↳Redirected from
"What are quantum numbers?"
1 Answer
Jun 23, 2014
Quantum numbers can be used to describe the quantum state of an electron.
Explanation:
Quantum numbers can be used to describe the quantum state of an electron.
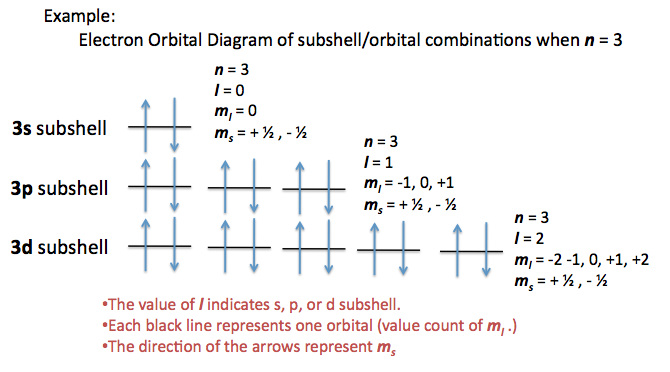
There are four quantum numbers for atoms:
-
#n = 1, 2, 3, . . . # - principal quantum number; describes the energy level. -
#l = 0, 1, 2, . . . , n - 1# - angular momentum quantum number; describes the shape of the orbital.
#0 harr s, 1 harr p, 2 harr d, 3 harr f, . . . # , etc. The ordering is#s,p,d,f,g,h,i,k, . . . # . -
#m_l = {-l, -l+1, . . . , 0, . . . , l-1, l}# - magnetic quantum number; corresponds to each unique orbital in the sublevel specified by#l# , and there are#2l+1# such values. #m_s = pm1/2# - spin quantum number; describes the spin (up/down)
A given orbital is labeled as an