What is the chemical formula of a diamond?
↳Redirected from
"What is the chemical formula of a diamond?"
1 Answer
Jan 27, 2014
The answer is simple:
Explanation:
Diamond is one form of carbon; the other is graphite. To distinguish them, we write:
-
diamond: C(s,diamond)
-
graphite: C(s,graphite)
The s stands for solid.
Both diamond and graphite are allotropes of carbon. There are other exotic allotropes of carbon (graphenes and fullerenes among them) but they are much less common.
A diamond consists of a giant three-dimensional network of carbon atoms.
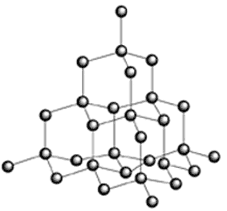
In effect, a diamond is one giant molecule. All we can do is write its empirical formula, which is

