Question #23ae1
2 Answers
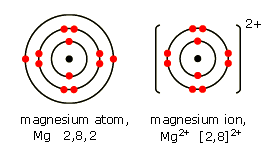
A magnesium atom has atomic number 12, so 12 protons in the nucleus and therefore 12 electrons. These are arranged 2 in the innermost (n=1) shell, then 8 in the next (n=2) shell, and the last two in the n=3 shell. Therefore a magnesium atom is [2,8,2]
The magnesium ion

Explanation:
Your starting point for finding the electron configuration of the magnesiu mcation,
You know that magnesium is located in period 3, group 2 of the periodic table, and that it has an atomic number equal to
This means that a neutral magnesium atom will have a total of
#"Mg: " 1s^2 2s^2 2p^6 3s^2#
Now, when the magnesium cation is formed, two electrons, hence the
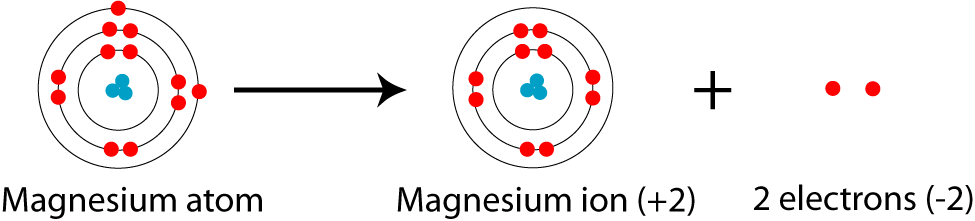
These electrons, which are called valence electrons, come from magnesium's outermost energy level. As you can see, magnesium has its outermost electrons located on the third energy level,
This means that when the cation is formed, its electron configuration will look like this
#"Mg: " 1s^2 2s^2 2p^6 color(red)(cancel(color(black)(3s^2))) implies "Mg"^(2+): 1s^2 2s^2 2p^6#
Using noble gas shorthand notation, you will get
#"Mg: " ["Ne"]color(red)(cancel(color(black)(3s^2))) implies "Mg"^(2+): ["Ne"]#