What is produced when #CH_3CONH_2# reacts with NaOBr? Is NaOBr a product of (or equivalent to) #Br_2 + NaOH# which are used in the Hoffmann bromamide degradation reaction?
1 Answer
Feb 27, 2015
The product is
This is an example of the Hofmann Degradation of primary amides to amines with one less carbon atom.
The reaction actually uses Br₂ + NaOH, but this is equivalent to using NaOBr, because the reaction produces NaOBr in situ.
The reaction involves several steps.
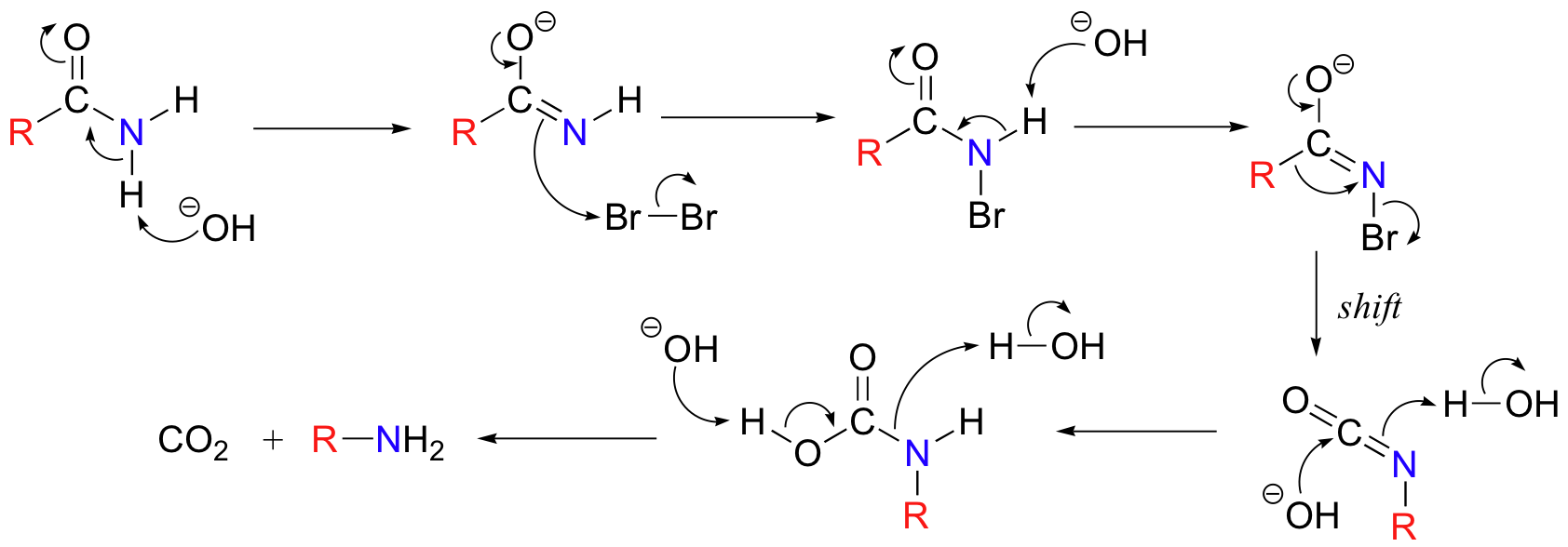
- The base abstracts an N-H proton, forming the conjugate base of the amide.
- The anion reacts displaces Br⁻ from a Br₂ molecule, forming an N-bromoamide.
- The base abstracts a proton from the N-bromoamide, forming another anion.
- The bromide ion leaves, and the alkyl group migrates to the N atom, forming an isocyanate.
- The base-catalyzed addition of water forms a carbamate.
- The carbamate acid spontaneously loses CO₂, forming an amine with one less carbon atom.


