What are two possible resonance structures for benzene?
1 Answer
There are only two possible resonance structures of the Benzene ring.
The double bonds (there are three in total) just move one place forward or backward, whichever way you want to look at it.
Image from: 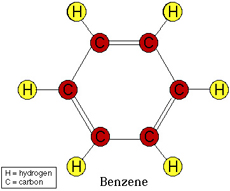
As you can see in the diagram the double bonds (two lines connecting the C's) are in a specific place, so the other resonance structure would be where the double bond is at the bottom and the two top edges of the hexagon.
*Double bonds must always be in conjugation, meaning there can't be a double bond next to a double bond, because then Carbon would not satisfy the four bond rule.
Hope I helped :)


