Question #e866b
1 Answer
An experiment performed by Robert Millikan.
Explanation:
Millikan determined the charge on an electron with this experiment. He put a charge on a tiny drop of oil, and measured how strong an applied electric field had to be in order to stop the oil drop from falling. If we analyze the experiment step by step;
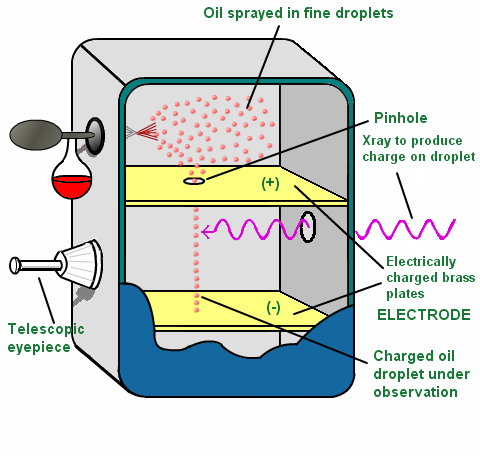
- He sent oil drops in a closed system.
- By using atomiser, he divided droplets into tiny particles.
- After passing of tiny droplets from a hole, he sent alpha rays.
- He seperated (+) and (-) charged particles.
- (-) particles were held by tiny droplets.
- He applied on electrical field with high voltage.
- (-) charged oil drops were suspended and the charge and mass of oil drops were calculated.


