How do neutrons help with the electromagnetic forces in the nucleus?
1 Answer
Neutrons are invisible to the electromagnetic force, and help hold the nucleus together.
Explanation:
Neutrons are neutrally charged particles, so they do not interact with the electromagnetic force. They are important in the nucleus of atoms because they do interact with the strong force.
 )
)
The strong force is the force that holds protons and neutrons together in the nucleus. If the nucleus were only composed of protons, then electrostatic repulsion would tear the atom apart.
Since neutrons do not have a charge, they can provide enough strong force to hold the protons together. Most stable atoms have a roughly 1-1 ratio of protons and neutrons.
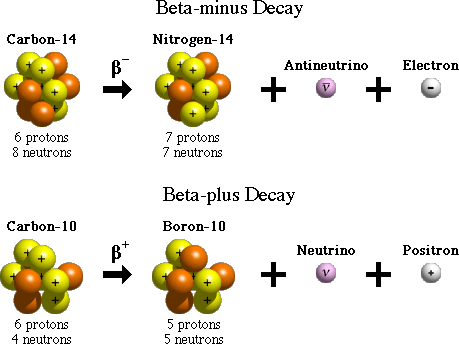
Sometimes, isotopes can be created that have uneven numbers of protons and neutrons. Nature solves this problem with the weak force by converting protons into neutrons, or neutrons into protons, allowing the extra charge to be flung away from the atom in the form of a positron or electron.