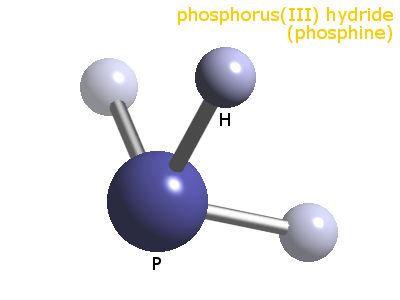
There are #5+3# valence electrons for which to account; and this gives 4 electron pairs arranged the central phosphorus atom. These assume a tetrahedral geometry, however, one of the arms of the tetrahedron is a lone pair, and geometry descends to trigonal pyramidal with respect to phosphorus.
The #/_H-P-H# #~=# #94""^@#, whereas that for #/_H-N-H~=105""^@#. This difference in bond angles between the homologues is not readily rationalized, and beyond the scope of even a third year inorganic chemist. This bond angle discrepancy is also observed for #H_2S# versus #H_2O#.
So, the shape is PYRAMIDAL :
 )
)
Hope it Helps :)
 )
) 

