How do you determine the electron configuration for the f block?
1 Answer
Nov 24, 2017
See below.
Explanation:
It's easier to understand how the f orbital fills using the long periodic table without the lanthanides and actinides removed:
![https://www.av8n.com/physics/img48/pt-block-fullpng]
(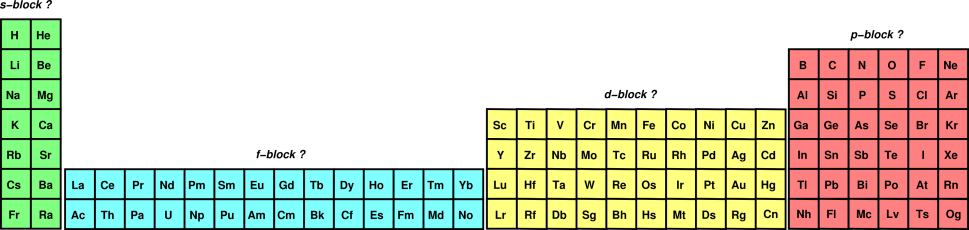 )
)
The f orbital will be 2 energy levels behind the row it's in, like how the d orbital in row 4 is 1 energy level behind (like in
The way you determine the number of electrons in an f orbital is the same way as the s, d, and p orbitals: count from left to right in the f block.
For example, La has 1 electron in the f orbital, Ce has 2, Pr has 3, Yb has 14, etc.
Hope this helped!

