How do molecules move in a solid?
1 Answer
In solids, the molecular motion between the particles is "vibratory" ie. they vibrate about their mean positions.
Explanation:
![http://www.mrreguinho.com/home/science/solids-and-liquids?tmpl=%2Fsystem%2Fapp%2Ftemplates%2Fprint%2F&showPrintDialog=1]
( )
)
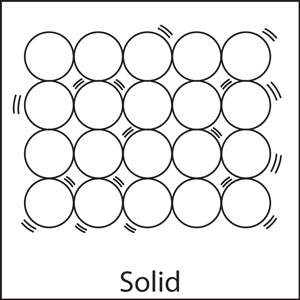
As the above diagram shows, the particles in solids are tightly packed. So it is not possible for them to have random, free motion. They vibrate back and forth, but the vibrations can't be detected by human senses.
The molecular forces between solid molecules are very strong, thus keeping them tightly packed and preventing them from moving freely.


