Question #09099
1 Answer
Element
Explanation:
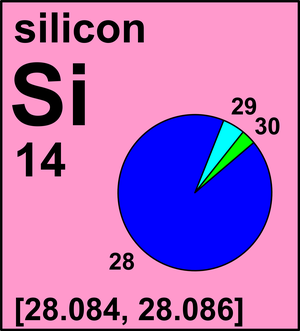
The idea here is that you need to use the atomic masses of the isotopes and their respective abundances to find the average atomic mass of element
Even without doing any calculations, you can guess that the average atomic mass of elemnt
Its high abundance tells you that it contributes most to the average atomic mass, so you can expect the atomic mass of element
You can use fractional abundances to calculate the contribution each isotope has to the average atomic mass
#"For " ""^28"X":" " 92.23/100 * "27.977 amu" = "25.80319 amu"#
#"For " ""^29"X":" "4.67/100 * "28.976 amu" = "1.35318 amu"#
#"For " ""^30"X":" "3.10/100 * "29.974 amu" = "0.92919 amu"#
The average atomic mass of the element will be the sum of each isotope's contribution
#"avg. atomic mass" = sum"isotope contribution"#
This means that you have
#25.80319 + 1.35318 + 0.92919 = color(green)("28.08556 amu")#
A quick look in the periodic table will reveal that element