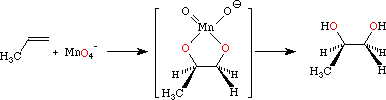
The mechanism involves a cyclic manganate ester, so the addition is stereospecific.
(a) Propene
#"CH"_3"CH=CH"_2 stackrelcolor (blue)("alk. MnO"_4^-)(→) "CH"_3stackrelcolor (blue)(✳)("C")"HOHCH"_2"OH"#
Note that the starred carbon atom is chiral, so the product is a racemic mixture of (#R#)- and (#S#)-propane-1,2-diol.
(b) cis-But-2-ene
#"CH"_3"CH=CHCH"_3 stackrelcolor (blue)("alk. MnO"_4^-)(→) "CH"_3stackrelcolor (red)(✳)("C")"HOH"stackrelcolor (red)(✳)("C")"HOHCH"_3#
Here we have two chiral carbons.
If you replace the wedged #"H"# atom in the diagram above with a #"CH"_3# group, you have the mechanism for the reaction with cis-but-2-ene.
Note that the two #"CH"_3# groups are both "in front", so the molecule has an internal plane of symmetry.
The product is meso-butane-2,3-diol.
(c) trans-But-2-ene
#"CH"_3"CH=CHCH"_3 stackrelcolor (blue)("alk. MnO"_4^-)(→) "CH"_3stackrelcolor (red)(✳)("C")"HOH"stackrelcolor (red)(✳)("C")"HOHCH"_3#
(the same equation)
But here the second #"CH"_3# group replaces the #"H"# atom on the dashed bond.
Both #"C"# atoms are chiral, but there is no longer a mirror plane.
The product is a racemic mixture of (#2R,3R#)- and (#2S,3S#)-butane-1,2-diol.

