#"CO"_2# has one carbon atom and two oxygen atoms, so it is called carbon dioxide. It's a gas you can find anywhere on Earth that has air, and it's why the pH of everyday water open to the air is less than #7# (it dissolves in water to make some #"H"_2"CO"_3#, an acid in soda).
#:stackrel(..)"O"="C"=stackrel(..)"O":#
#"C"_6"H"_12"O"_6# corresponds to many different compounds, but I presume you mean glucose (a six-carbon sugar), which plants make from photosynthesis. It's also made and/or utilized in our bodies, in for example, the citric acid cycle, glycolysis, etc.
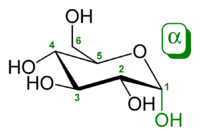
The following structure has the name #alpha#-D-glucopyranose:
 )
)
It does break open in water, but it exists primarily (#99%#) as this ringed structure in water.
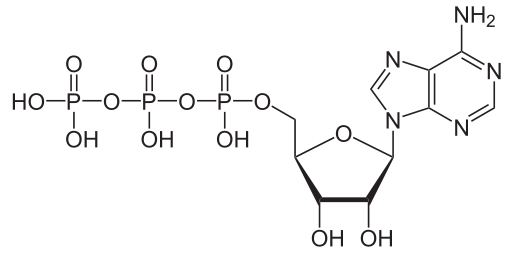
#"ATP"#, or adenosine triphosphate, is often referred to as the energy carrier in the cells of many organisms. It holds three phosphate groups, and essentially releases and re-binds to one or two phosphate groups to transport energy.
 )
)
 )
)  )
) 
