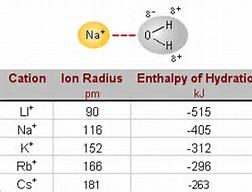
What do we call the energy evolved when a gaseous metal cation is hydrated by bulk water?
1 Answer
Jul 22, 2017
So far as I know,
Explanation:
So far as I know,
Where
This is certainly measurable for a given series of CATIONS (if we keep the anion constant....) and hydration energy increase by an inverse proportion to the size of the ion.....