What are the conditions for an exothermic vs. an endothermic reaction?
1 Answer
Jan 16, 2018
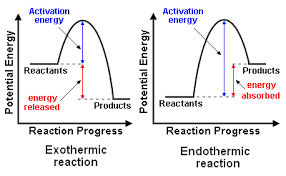
In case of an exothermic reaction,the heat content of products is less than substrates,so during this reaction energy is released.
but in an endothermic reaction,the heat content of products is more than substrates,hence it absorbs energy.
#a+b -> c+d#
this reaction will be exothermic if
#delH_c + delH_d < delH_a+delH_b#
and endothermic if
#delH_c + delH_d > delH_a+delH_b# (where
#H# stands for heat content i.e ENTHALPY)


