Do all of the following molecules contain at least one bond angle at 120 degrees: SeS3, SeS2, PCl5, TeCl4, ICI3, and XeCl2?
1 Answer
No, because two of the molecules have no bond angles at 120 °.
Explanation:
To answer this question, you must draw the Lewis structures of the compounds and determine their VSEPR shapes.
The Lewis structure of
(from www.homeworklib.com)
This is an
All bond angles in a trigonal planar molecule are approximately 120 °.
The Lewis structure of
This is an
Its electron geometry is trigonal planar.
The bond angle in an
The Lewis structure of
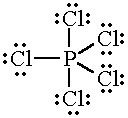
This is an
The equatorial bond angles in a trigonal bipyramidal molecule are all 120 °.
The Lewis structure of

This is an
Its electron geometry is trigonal pyramidal.
The equatorial bond angle between the equatorial
The Lewis structure of
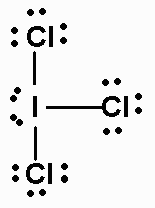
This is an
Its electron geometry is trigonal pyramidal, and its molecular geometry is T-shaped.
The
The Lewis structure of
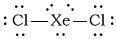
This is an
Its electron geometry is trigonal pyramidal, with the lone pairs in the equatorial positions.
The molecule is linear, with a

