How can I identify and draw the optical isomers for the isomers of #[Cr(H_2O)_3Cl_3 ]^+#?
1 Answer
Jun 5, 2015
There are two isomers: fac and mer.
There are three ligands of one type and three ligands of another type.
It is classed as an
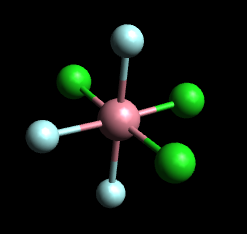
In the facial (fac) isomer, each set of three identical ligands occupies one face of the octahedron.
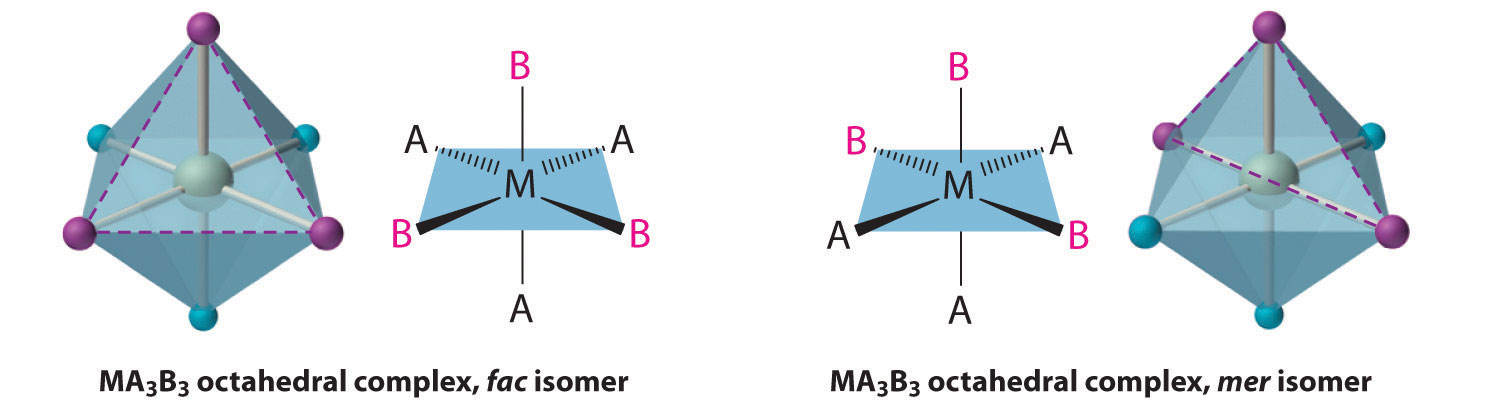
In the meridional (mer) isomer, each set of three identical ligands is arranged in a T-shape in a plane that includes the metal atom.
We can make a model in which green spheres represent
Then the model below represents fac-
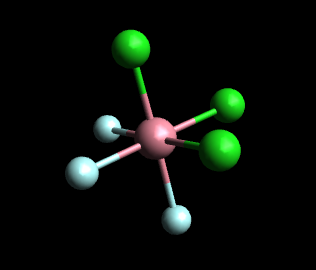
And the model below represents mer-