How do London dispersion forces work?
1 Answer
Explanation:
For a given, NEUTRAL atom, a transient UNEQUAL electronic distribution might result in a transient UNEQUAL electronic distribution in a neighbouring atom....and this transient UNEQUAL electronic distribution in the neighbouring atom may result in a transient UNEQUAL electronic distribution in another atom. And so on, and so on....
And this intermolecular force is presumed to operate for the
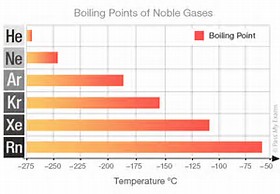
Anyway look up the boiling points of the Noble Gases....

