How do oxidation numbers vary with the periodic table?
1 Answer
The only consistent variation of oxidation numbers with the Periodic Table is that, in their compounds, Group 1 metals are always +1 and Group 2 metals are always +2.
Consider a carbon atom, for example. Because of the way that we count electrons for oxidation number purposes, carbon can have any of the oxidation numbers -4, -3, -2, -1, 0, +1, +2, +3, or +4.
This is because one of the rules is that electrons that are shared between nonidentical atoms belong entirely to the more electronegative atom.
Let’s apply this rule to the carbon atoms in acetic acid.
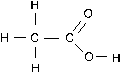
The left hand C is attached to three (less electronegative) H atoms and the other C atom. It gets all six electrons in the C-H bonds and only one electron from the C-C bond. This gives carbon 7 valence electrons, which is 3 more than it normally has. The oxidation number of this C atom is -3.
The right hand C atom loses all of its shared electrons to the more electronegative O atoms. It keeps the one electron from the C-C bond. Since it has officially lost 3 electrons, its oxidation number is +3.

