How do resonance structures affect acidity?
1 Answer
Short answer: Resonance structures that stabilize a conjugate base will increase the acidity.
Explanation:
Consider the acidities of methanol and acetic acid.
CH₃OH + H₂O ⇌ CH₃O⁻ + H₃O⁺;
CH₃COOH + H₂O ⇌ CH₃COO⁻ + H₃O⁺;
Why is acetic acid
Answer: Here is my explanation.
In methoxide ion, the negative charge is localized (concentrated) on the oxygen atom.
Resonance stabilizes both acetic acid and acetate ion,
In acetic acid, the stabilization is small because the resonance contribution involves separation of charge.
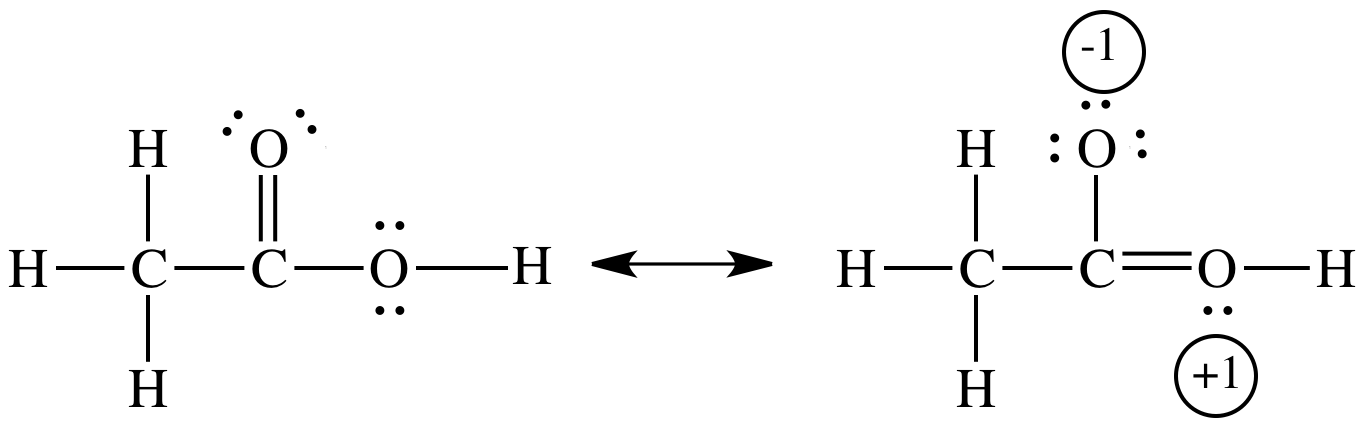
In acetate ion, there is no separation of charge.
Instead, the negative charge is delocalized (spread out) over three atoms. This delocalization produces a lower-energy state.
If the products of a reaction are more stable than the reactants, the position of equilibrium will lie to the right.
So, the resonance stabilization of acetate ion makes acetic acid more acidic than methanol.
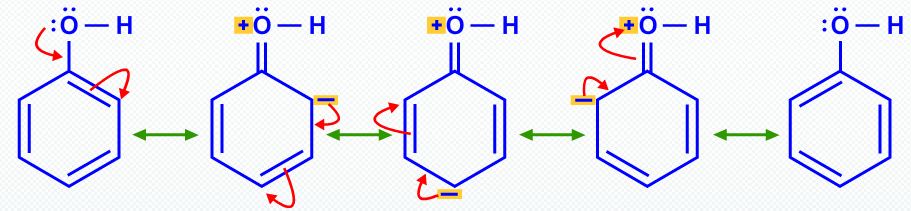
In the same way, resonance makes phenol more acidic than ethanol.
There is no resonance stabilization in the ethoxide ion.
Resonance stabilizes both phenol and phenoxide ion by delocalization of electrons into the ring.
However, thisδocalization in phenol involves separation of charge and makes the oxygen atom positive.
The same delocalization in phenoxide ion provides much more stabilization because there is no charge separation.

The position of equilibrium lies to the right.
Thus, phenol is