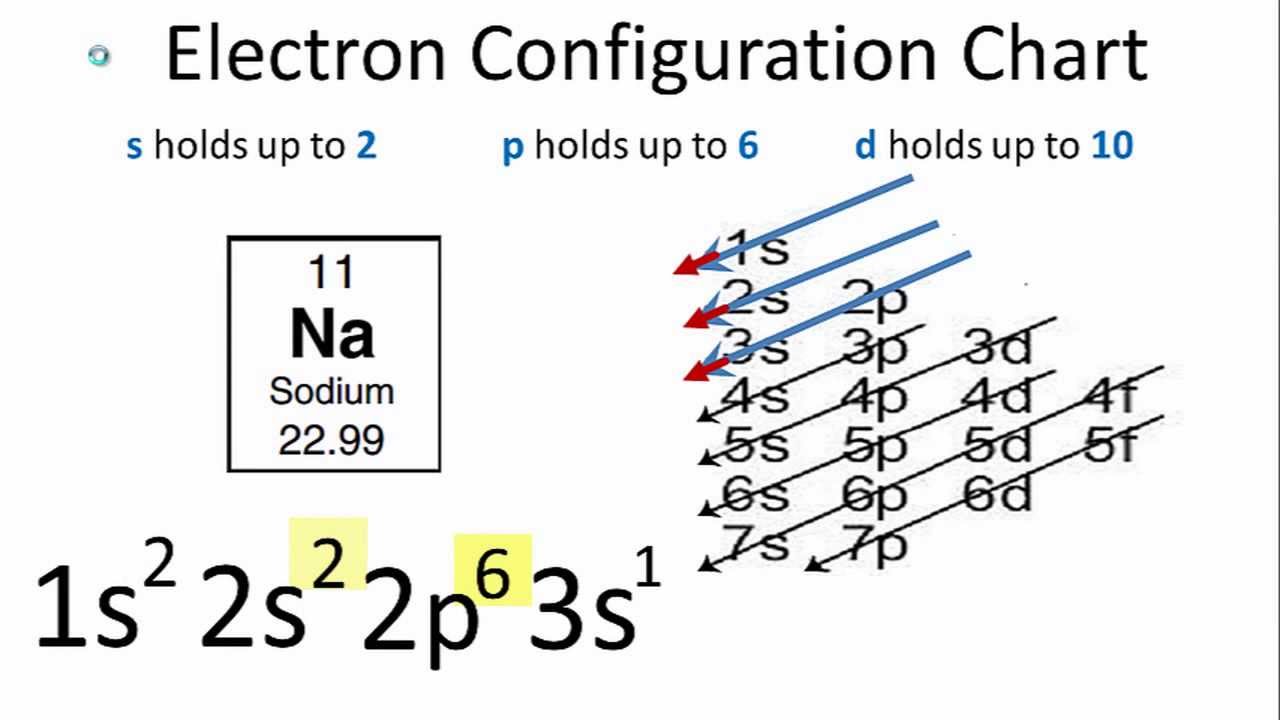
#Z# gives the number of nuclear protons, and the number of electrons that we have to distribute according to a given scheme......
 )
)
The scheme is not perfect, and it falls down for chromium metal, which is #Z=24#, and gives #1s^2 2s^2 2p^6 3s^2 3p^6 3d^5 4s^1# rather than but #1s^2 2s^2 2p^6 3s^2 3p^6 3d^4 4s^2#, but you can learn this and several other exceptions.......
The features the filling of the #"4s-orbitals"# BEFORE the #"3d-orbitals"# begin to be filled.....And thus the first-row transition metals typically have a FULL #4s# shell, cf. iron, #Z=26#, and gives #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^6 #
And given the position of the element on the Periodic Table, #"s-block"#, #"p-block"#, #"d-block"#, usually you can define the valence configuration without difficulty.
Anyway, this is the knowledge expected of an undergraduate student. I don't know whether you are there or at A-level.
 )
) 
