How does pressure affect the reaction rate?
1 Answer
Apr 26, 2014
Increasing the pressure on a reaction involving reacting gases increases the rate of reaction. Changing the pressure on a reaction that involves only solids or liquids has no effect on the rate.
If you increase the pressure of a gas, you squeeze it into a smaller volume.
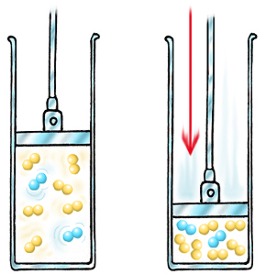
In order for any reaction to happen, the particles must first collide. If there are more molecules in a smaller volume, the chances of collision are greater, and the rate increases.
The volume of solids and liquids does not change with pressure.
Increasing the pressure on solids and liquids has no effect on the reaction rate.

