How is nitrogen-14 an anion?
Since it has 7 protons, neutrons, and electrons, wouldn't it not be an anion since it does not have a negative electric charge?
Since it has 7 protons, neutrons, and electrons, wouldn't it not be an anion since it does not have a negative electric charge?
1 Answer
An atom of nitrogen-14 is not an anion.
Explanation:
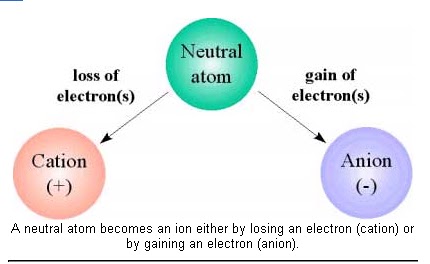
First and foremost, if an atom has equal numbers of protons inside the nucleus and of electrons surrounding the nucleus, then it is electrically neutral, i.e. it is neither a cation nor an anion.
In order for an atom to be an anion, it must have more electrons surrounding the nucleus than protons inside the nucleus.
You know that
#"net charge" = "no. of protons " - " no. of electrons"#
This shows that equal numbers of protons and electrons correspond to a net charge of
If you have
#"no. of electrons " > " no. of protons"#
then you get
#"net charge" < 0#
In this case, the atom is an anion because it carries a negative net charge.
Also, keep in mind that the identity of the isotope is irrelevant when it comes to the net charge of the atom.
In this case, nitrogen-14 denotes the isotope of nitrogen that has
In order for nitrogen-14,
In this case, you have
#"net charge" = 7 - 10 = -3#
which means that the anion carries a
#""^14"N"^(3-)#
As a final note, don't forget to review the difference between neutral atoms, cations, and anions.