Regarding E2 reactions, predict the stereochemistry of the bromoalkene that would result from the loss of one mol of #HBr# from meso- and from (±)-1,2-dibromo-1,2-diphenylethane. Which reaction should be slower? Why?
1 Answer
Explanation:
In
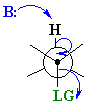
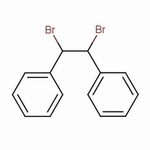
1,2-Dibromo-1,2-diphenylethane
The structure of 1,2-dibromo-1,2-diphenylethane is
E2 elimination of meso-1,2-dibromo-1,2-diphenylethane
The Newman projection of the meso isomer is
We know that the structure is meso because the order of groups
In the transition state, the
This gives a large activation energy and make for a slow reaction.
The product will have the two phenyl groups on the same side, giving an
(from chemwiki.ucdavis.edu)
E2 elimination of (±)-1,2-dibromo-1,2-diphenylethane
The Newman projection for one of the enantiomers is
We know that the structure is chiral because the order of groups
In the transition state, the
The activation energy will be less, so this will be a faster reaction.
The product will have the two phenyl groups on opposite sides, giving a
(from chemwiki.ucdavis.edu)

