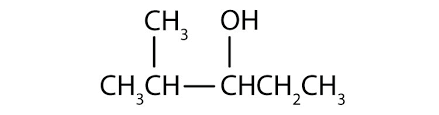
What are primary and secondary alcohols?
1 Answer
Primary alcohols have a hydroxyl on their end, while in secondary alcohols the hydroxyl is somewhere along the carbon chain's length.
Explanation:
Primary alcohols are where the hydroxyl (
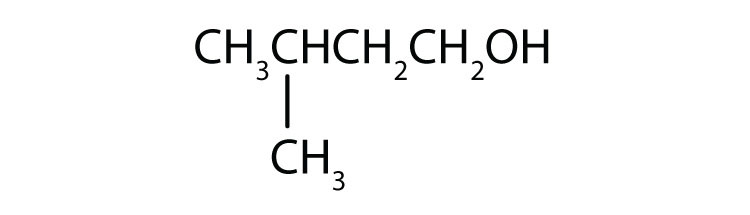
Secondary alcohols are where the hydroxyl is attached to an alkyl that is attached to more than one other alkyl, so includes the group