What are some examples of molecular orbitals?
1 Answer
The simplest molecular orbitals are the σ and σ orbitals formed by the overlap of atomic s* orbitals.
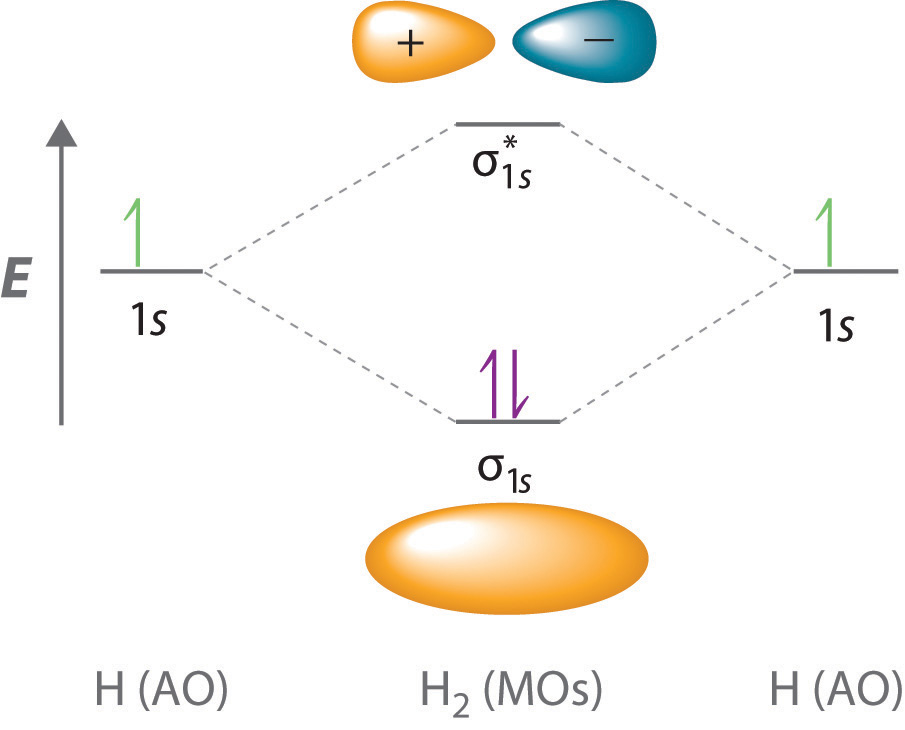
We also have σ(2p) and σ*(2p) orbitals formed by the end-on overlap of 2p orbitals.

In alkanes such as ethane we can also have σ orbitals formed by the overlap of atomic s and sp³ atomic orbitals in C-H bonds. The C-C bonds form by the overlap of sp³ atomic orbitals.

Molecular π orbitals form by the sideways overlap of atomic p orbitals.
http://www.dlt.ncssm.edu/tiger/diagrams/[bonding](http://socratic.org/chemistry/bonding-basics/bonding)/p_pi_bonding.jpg
Then we can have extended π orbitals. The four atomic orbitals on the C atoms in buta-1,3-diene overlap to form the four π orbitals.

These are only a few of the many molecular orbitals that are possible.

