What element in Bohr form is 1s2 2s2 2p6 3s2 3p4?
1 Answer
The element you're looking for is sulfur, or
Here's how you can use an element's electron configuration to determine its identity.
If you add all the electrons decribed in this electron configuration you'll get
For a neutral atom, the number of electrons it has will match the number of protons in its nucleus, i.e. its atomic number. This means that the element you're looking for will have its atomic number equal to 16. According to the periodic table, this element is sulfur.
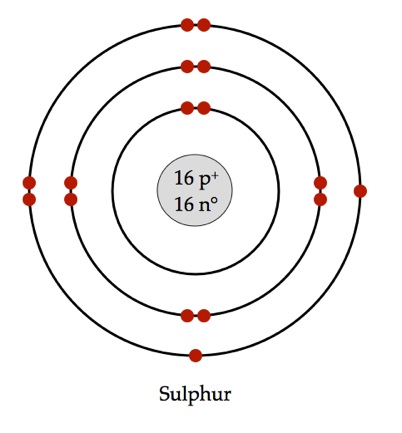
Read more on sulfur's Bohr model here:
http://socratic.org/questions/what-is-the-bohr-model-for-sulfur

