What intermolecular forces are present in #CH_4#?
2 Answers
Just London (dispersion forces).
Because methane is a non-polar molecule it is not capable of hydrogen bonding or dipole-dipole intermolecular forces.
The only intermolecular forces in methane are London dispersion forces.
The major intermolecular forces would be dipole-dipole forces and London dispersion forces.
The electronegativities of C and H are so close that C-H bonds are nonpolar. There are no bond dipoles and no dipole-dipole interactions.
Even if the molecule had polar C-H bonds, the symmetry of molecule would cause the bond dipoles to cancel. The molecule would still be nonpolar. It is a blob with no positive or negative ends.

The only forces left to consider are London dispersion forces.
In a nonpolar molecule, electrons are always moving. At any instant, they might be at one end of the molecule. This would instantaneously create a temporary dipole, making that end negative and the other end positive.
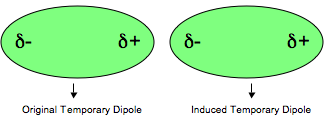
The positive charge attracts the electrons in an adjacent molecule. This temporary attractive force is the London dispersion force
The London dispersion forces are so weak that methane does not condense to a liquid until it cools to −161.5 °C.


