What is a differential scanning calorimeter?
1 Answer
A differential scanning calorimeter is a special calorimeter that heats a sample and a reference at the same rate. It measures the difference in the amount of heat required to increase the temperature of sample and reference as a function of temperature.
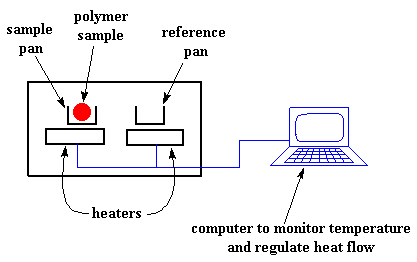
Differential scanning calorimetry (DSC) is often used to study polymers.
You heat a sample and a reference so their temperatures increase at the same rate. When the sample undergoes a phase transition, a different amount heat will flow to the sample than to the reference. You plot the difference in heat flow as a function of temperature.
MELTING:
The melting of a solid is endothermic. The extra heat flow to maintain the temperature appears as a peak on the plot.
CRYSTALLIZATION:
When the sample crystallizes, less heat flows to the sample. This appears as a dip in the plot.
GLASS TRANSITION:
After a certain temperature, a polymer may undergo a glass transition. Its heat capacity increases.
A complete plot often looks something like this:


