What is a reaction rate constant?
1 Answer
The Reaction Rate for a given chemical reaction is the measure of the change in concentration of the reactants or the change in concentration of the products per unit time. A Reaction Rate Constant, k, quantifies the rate of the chemical reaction.
The rate is usually measured by looking at how fast the concentration of one of the reactants is falling at any one time. For example, suppose you had a reaction between two substances A and B. Assume that at least one of them is in a form where it is sensible to measure its concentration - for example, in solution or as a gas.
A + B -------> Products
For this reaction you could measure the rate of the reaction by finding out how fast the concentration of, say, A was falling per second.
This is called the rate equation for the reaction:
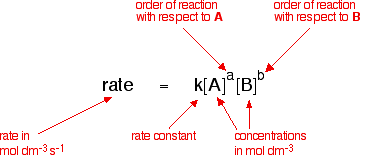
The concentrations of A and B have to be raised to some power to show how they affect the rate of the reaction. These powers are called the orders of reaction with respect to A and B.
The rate constant isn't actually a true constant! It varies if you change the temperature of the reaction, add a catalyst or change it. The rate constant is constant if the only thing you are changing is the concentration of the reactants.


