What is halogenation?
2 Answers
Halogenation is the replacement of a hydrogen atom by a halogen atom in a molecule.
Halogens is the group name that is given to fluorine, chlorine, bromine and iodine. Since these elements have very similar behaviour, they are often treated as a group. The term 'halogenation' can thus refer to replacing any number of hydrogen atom with each and any of the members of the group.
The product resulting from halogenation will have quite distinct properties from the start compound.
Example:
Methane
If you chlorinate (=halogenate with chlorine) you get different substances like
named mono-, di-, tri- and tetra-chloro-methane (the tri-version also known als chloroform)
The completely chlorinated compound
There are several processes to halogenate carbon compounds.
One other group of products are the result of mixed halogenation, i.e. different halogens are used, for instance chlorine and fluorine. This leads to chloro-fluoro-carbons (CFC's), of which
Halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance.
Explanation:
There are two types of halogenation.
Halogen Addition
An example is the addition of bromine to ethene.
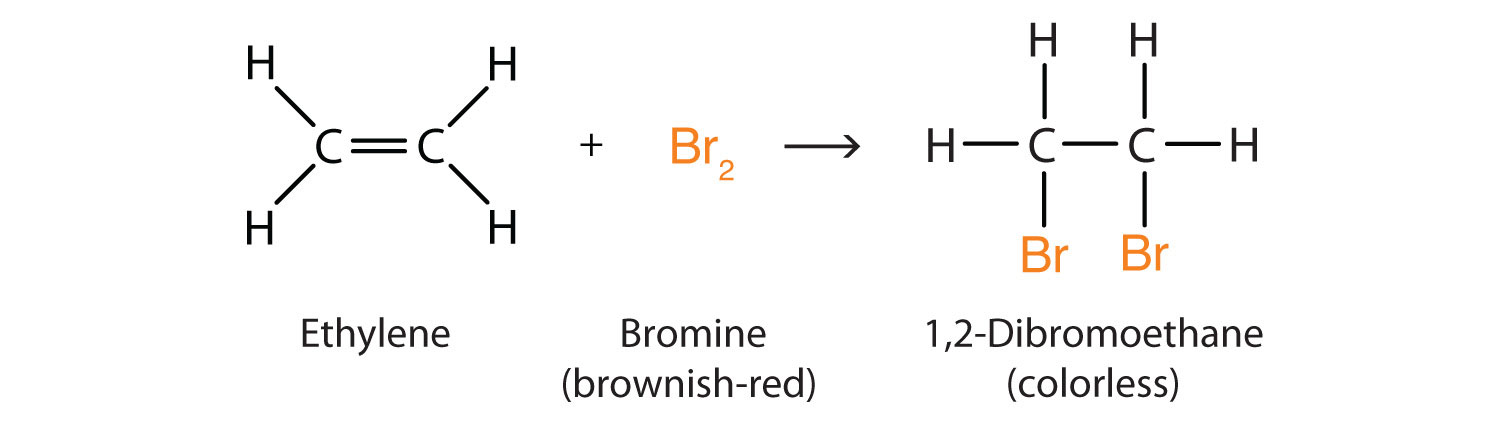
Halogen substitution
Halogens react with alkanes under the influence of heat or light to form alkyl halides.
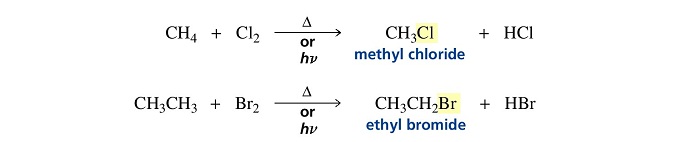
The halogen atom replaces a hydrogen atom in the alkane, so this is a substitution reaction.
Aromatic compounds undergo halogen substitution reactions in the presence of Lewis acids.

Here's a video on the halogenation of alkanes.


