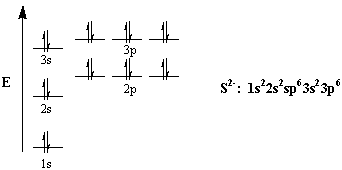What is the electron configuration for #S^(2-)# ion?
1 Answer
Explanation:
A good starting point when looking for the electron configuration of an ion is the electron configuration of the neutral atom.
In your case, the neutral atom is sulfur,
The electron configuration of a neutral sulfur atom will thus be
#"S: " 1s^2 2s^2 2p^6 3s^2 3p^4#
Now, the sulfide anion,
As you can see in the configuration of the neutral atom, these two electrons will be added to the 3p-orbitals, which can hold a maximum of six electrons between them.
The electron configuration of the sulfide anion will thus be
#"S"^(2-):color(white)(a) 1s^2 2s^2 2p^6 3s^2 3p^6#
The noble gas shorthand notation for the sulfide anion will use the electron configuration of neon, the noble gas that comes immediately before sulfur in the periodic table.
#"S"^(2-): color(white)(a)["Ne"] 3s^2 3p^6#