What is the electron configuration of Li+?
1 Answer
Explanation:
Your starting point here will be the electron configuration of a neutral lithium atom,
A quick look in the periodic table will reveal that lithium is located in period 2, group 1, and that it has an atomic number equal to
This means that a neutral lithium atom will have a total of
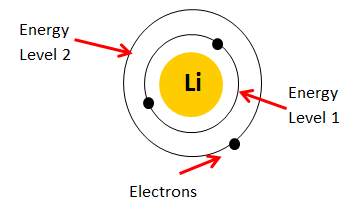
Its electron configuration will be
#"Li: " 1s^2 color(red)(2)s^1#
Now, the lithium cation,
This means that the electron configuration of the
#"Li"^(+): 1s^2#
To write this using noble gas shorthand notation, use the electron configuration of the noble gas that comes before lithium in the periodic table.
Helium,
#"He: " 1s^2#
This means that you have
#"Li"^(+): ["He"]#
Here the notation

