What is the empirical formula of H2O2?
2 Answers
The empirical formula is the simplest whole number ratio that defines constituent atoms in a species.
Explanation:
Given the above definition the empirical formula of hydrogen peroxide is simply
What are the empirical formulae of benzene, ethylene, and acetylene; the molecular formulae are
The empirical formula for the molecular formula
Explanation:
The empirical formula of a compound is the lowest whole number ratio of elements in the compound. This ratio becomes the subscripts for the empirical formula.
The molecular formula
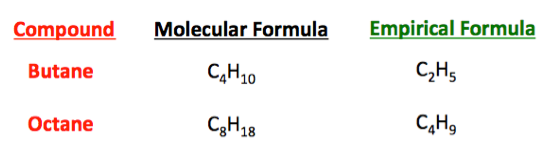
Sometimes the molecular and empirical formulas are the same.



