What is the likeliest m/z value for the base peak in the mass spectrum of 3-methylpentane?
1 Answer
May 6, 2015
The most likely value for the base peak in the mass spectrum of 3-methylpentane is at
The structure of 3-methylpentane is
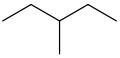
Mass spectroscopic fragmentation of hydrocarbons tends to occur at a branch.
So there are two possible fragmentation patterns:
- Loss of
#"CH"_3 ("M" -15)# .This will give a peak at#m"/"z =71# , corresponding to#"CH"_3"CH"_2"CH"^+"CH"_2"CH"_3# . - Loss of
#"CH"_3"CH"_2 ("M" - 29)# . This will give a peak at#m"/"z = 57# , corresponding to#"CH"_3"CH"^+"CH"_2"CH"_3# .
The peaks at 57 and 29 should be more intense than those at 71 and 15, because the ethyl cation (29) is more stable than the methyl cation (15).
The 2-butyl cation is more stable than the ethyl cation, so the base peak is probably at

