What is the molecular geometry around an atom that has 2 #sigma# bonds, 2 #pi# bonds, and 0 lone pair of electrons?
1 Answer
Linear.
Explanation:
The most important thing to realize here is that you need a sigma bond in order to have a pi bond.
Simply put, a double or a triple bond, the two types of bonds that contain at least one pi bond, also contain one sigma bond.
More specifically, single, double, and triple bonds contain
- one sigma and zero pi bonds
#-># single bond- one sigma and one pi bond
#-># double bond- one sigma and two pi bonds
#-># triple bond
This means that the central atom for your molecule will have
-
one single bond to one atom and one triple bond to a second atom
-
one double bond to one atom and one double bond to a second atom
In both cases, the central atom will form two sigma bonds and two pi bonds.
With this being said, what is the exact bonding for the central atom is actually irrelevant to determining its molecular geometry because both possible configurations are equivalent to two regions of electron density.
As you know, molecular geometry is derived from the hybridization of the central atom. In order to determine the hybridization of the central atom, you must count the regions of electron density that surround it.
These regions of electron density are
- a single, double, or triple bond
#-># all three count as one region of electron density - a lone pair of electrons**
In your case, you know that the central atom has no lone pairs attached, so right from the start you can say that it will be surrounded by two regions of electron density.
This means that the central atom will be
Here are two examples of how your molecule could look like
 )
)
This is the scenario in which the central atom is bonded to two other atoms via two double bonds.
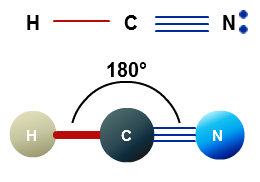
This is the scenario in which the central atom is bonded to two other atoms via a single bond and a triple bond.

