Why are closed shells more stable?
1 Answer
Closed shells are more stable because they have the highest ionisation energy in their periods.
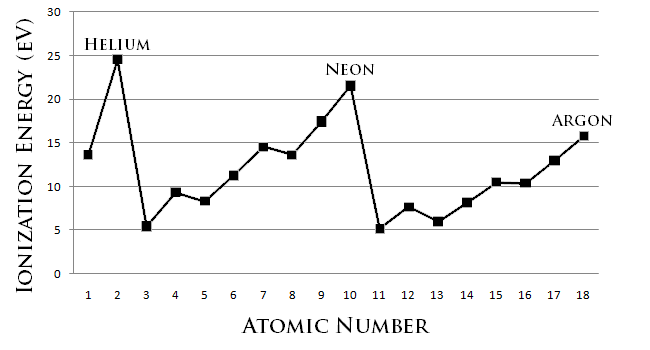
Stability means being unreactive. The most unreactive elements are those least willing to give up an electron (or least willing to gain one). You can see from the diagram that the noble gases, with full (closed) out shells have the highest ionization energies and so have the strongest attractions for their out shell electrons, so they are least willing to transfer or share electrons.
For completeness we ought to consider electron affinity too - the energy released when an atom gains an electron. The noble gases have the lowest electron affinities so are least able to attract an additional electron into their outer shell - another way of reacting.



