Why are hydrogen atoms omitted in bond line notation?
1 Answer
Oct 29, 2014
Organic compounds contain many hydrogen atoms.
It would be tedious to draw all the C-H bonds explicitly. And all those H atoms clutter up the structural formulas of large molecules.
So we leave them out. But we then have to remember that each carbon has enough H atoms to make its valence up to four.
Consider hexan-1-ol.
Its structural formula is
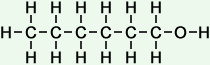
Its bond line structure is

The latter structure eliminates the drawing of 13 H atoms and 13 C-H bonds. And it gives a much simpler drawing of the molecular structure.

