Why can cyclohexane rings cause some complications with elimination reactions?
1 Answer
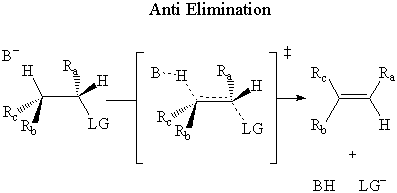
Cyclohexane rings can cause complications because E2 eliminations must be antiperiplanar.
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule.
An example is the dehydrobromination of bromoethane.

The H atom must come from the β carbon atom — the carbon next to the one bearing the leaving group.
Also, the bonds to the β H and to the leaving group LG must be antiperiplanar.
Consider the dehydrohalogenation of menthyl chloride, (
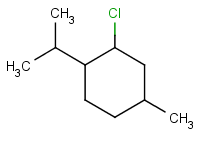
If we ignore stereochemistry, we identify β H atoms at C-1 and C-3. The major product should be the more stable Zaitsev product, 1-isopropyl-4-methylcyclohexene.

But this product forms in 0 % yield. Only the less stable Hofmann product forms, but slowly.

This makes sense if we consider the stereochemistry of menthyl chloride.
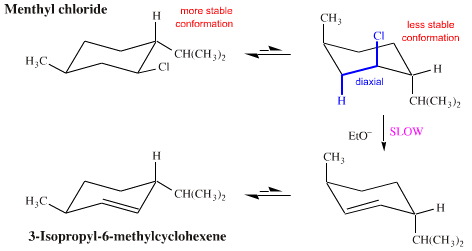
In the most stable chair form, the bulky isopropyl group must be in the equatorial position. This makes the Cl also equatorial.
The ring must flip to the less stable form. That makes both isopropyl and Cl axial.
But then only the β H atom at C-3 is axial and antiperiplanar to the Cl.
So the only observed product is 3-isopropyl-6-methylcyclohexene.

