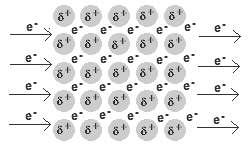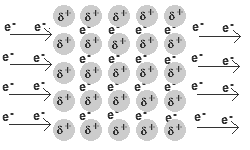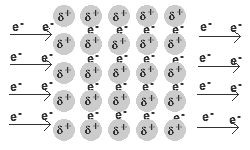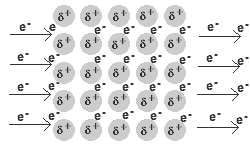Why do metallic compounds conduct electricity as a solid?
↳Redirected from
"What polyatomic ion has a positive charge?"
1 Answer
Apr 20, 2014
Compounds of metals do not conduct electricity as a solid, but metals are good conductors of electricity.
Explanation:
An electric current consists of the movement of charged particles.
Compounds of metals are salts. They consist of oppositely charged ions.
For example, NaCl consists of Na⁺ and Cl⁻ ions arranged in a crystal lattice.
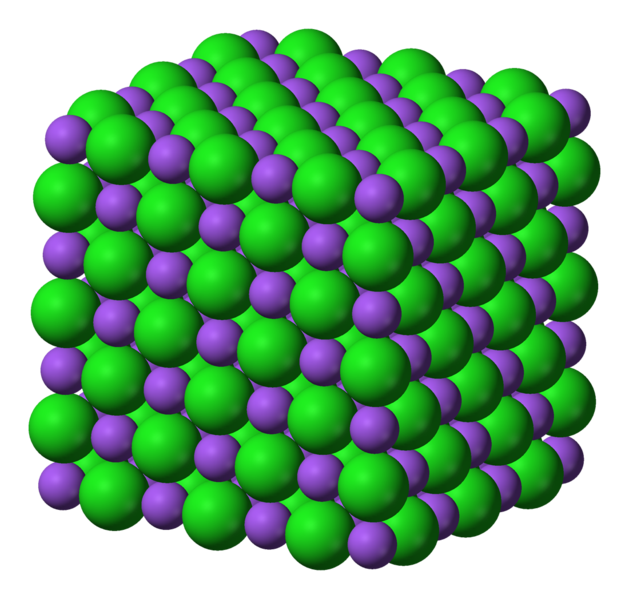
The ions in the crystal cannot move, so solid NaCl does not conduct electricity.
In a metal, the valence electrons are loosely held.
They leave their “own” metal atoms, forming a "sea" of electrons surrounding the metal cations in the solid.
The electrons are free to move throughout this electron sea.

The movement of electrons is an electric current. Thus, metals are good conductors of electricity.