Why do Van der Waals forces increase with the size of molecules?
1 Answer
There are two effects: (a) bigger atoms are more polarizable, and (b) bigger molecules have more electrons to polarize.
Polarizability
Polarizability is the ease of distorting an electron cloud.
A iodine atom is bigger than a chlorine atom. Its valence electrons are much further from the nucleus, so they are not tightly held.
A nearby dipole can distort the electron cloud of iodine much more than it can distort that of a smaller atom.

An iodine molecule has much stronger London dispersion forces than a chlorine molecule. That's why, at room temperature, chlorine is a gas, bromine is a liquid, and iodine is a solid.
Molecular Size
Even if the atoms are the same size, bigger molecules have stronger London dispersion forces. There are more electron clouds to distort.
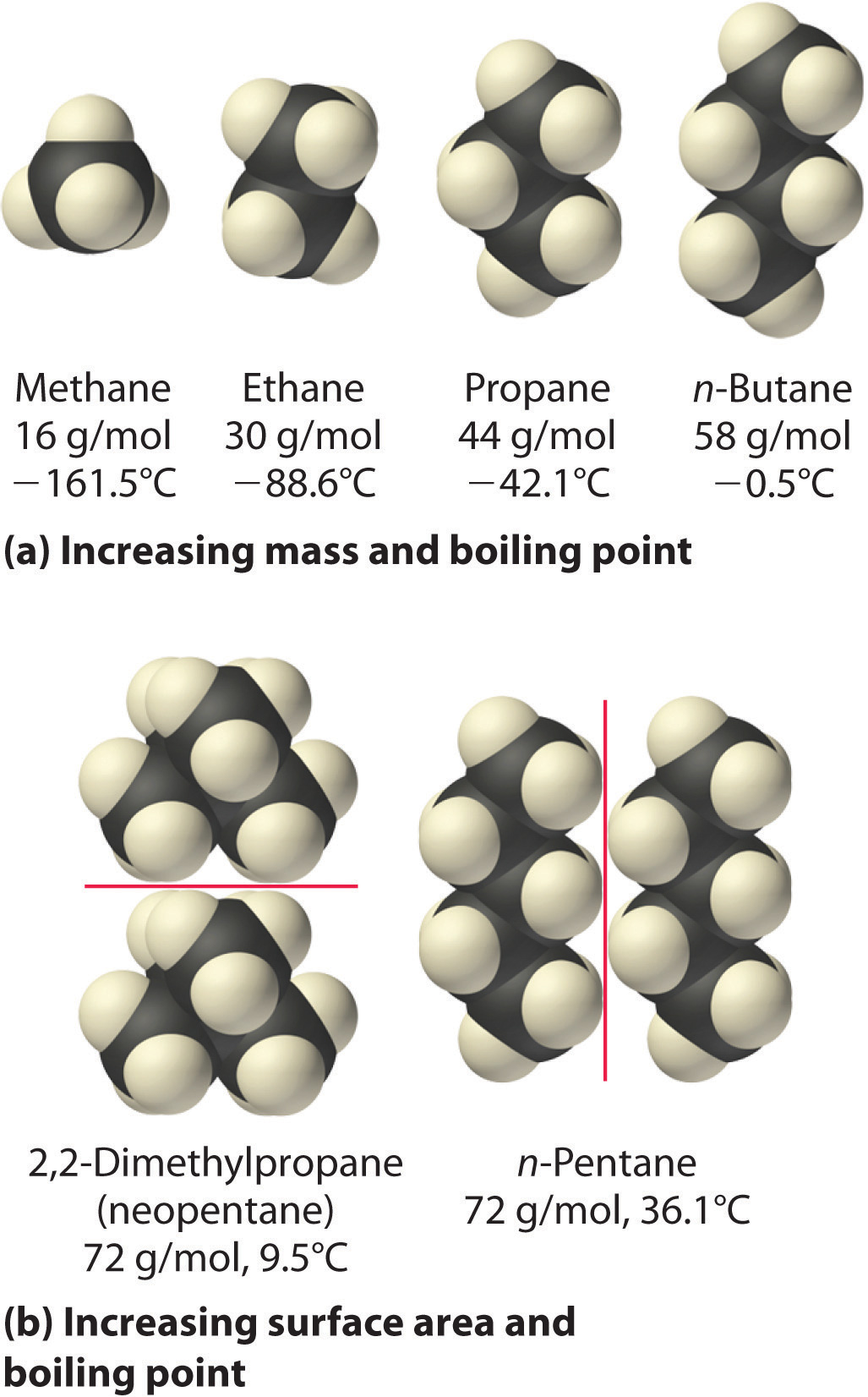
The hydrocarbons from methane to pentane contain only C and H atoms. As the chains get longer and snuggle up close to each other, there are more contact points between them. The attractions at each contact point add up.
That's why the boiling points increase on going from methane to pentane.

