WARNING! Long answer!
During respiration, glucose molecules are converted to other molecules in a series of steps.
They finally end up as carbon dioxide and water.
The overall reaction is
#"C"_6"H"_12"O"_6 + "6O"_2 → "6CO"_2 + "6H"_2"O" + "2805 kJ"#

The reaction is exothermic because the #"C=O"# and #"O-H"# bonds in the products are so much more stable than the bonds in the reactants.
Bond energy is the average energy needed to break a bond.
Some bond energies are:
#"C-C"# = 347 kJ/mol
#"C-H"# = 413 kJ/mol;
#"C-O"# = 358 kJ/mol;
#"O-H"# = 467 kJ/mol;
#"O=O"# = 495 kJ/mol;
#"C=O"# = 799 kJ/mol
We can view the process as breaking all the bonds in the reactants to separate the atoms and then re-combining the atoms to form the bonds in the products.
A glucose molecule has the formula
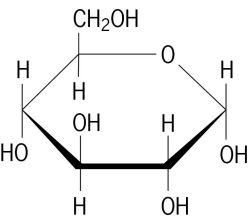
It contains 5 #"C-C"#, 7 #"C-H"#; 7 #"C-O"#, and 5 #"O-H"# bonds.
The 6 #"O"_2# molecules contain 6 #"O=O"# bonds.
The products contain 12 #"C=O"# and 12 #"O-H"# bonds.
The overall process is
#"5C-C"color(white)(l) + "7C-H" + "7C-O" +color(red)(cancel(color(black)("5O-H"))) + "6O=O" → "12C=O" + stackrelcolor(blue)(7)(color(red)(cancel(color(black)(12))))"O-H"#
or
#underbrace("5C-C"color(white)(l) + "7C-H" + "7C-O"+ "6O=O")_color(red)("break these bonds")→ underbrace("12C=O"color(white)(l) + "7O-H")_color(red)("form these bonds")#
The energy differences in kilojoules per mole are
#color(white)(l)"5C-C" +color(white)(l) "7C-H" + "7C-O"color(white)(l)+color(white)(l) "6O=O"color(white)(l) → "12C=O" +color(white)(l) "5O-H"#
#"5×347" + "7×413" + "7×358"+color(white)(l) "6×495"color(white)(l) → "12×799" + "7×467"#
#color(white)(l)1735color(white)(l) +color(white)(l)2891color(white)(l) +color(white)(l) 2506color(white)(l) +color(white)(ll) 2970 color(white)(m)→ color(white)(m)9588 color(white)(ll)+color(white)(l) 3269#
#color(white)(mmmmmmm)"10 102"color(white)(mmmmmmml) → color(white)(m) color(white)(mm)"12 857"#
#ΔH ≈ "(10 102 - 12 857) kJ/mol" = "-2755 kJ/mol"#
Almost 75 % of the energy released comes from formation of the stable #"C=O"# bonds in #"CO"_2#.



