An #s# orbital is a sphere. In two dimensions, we draw it as a circle.
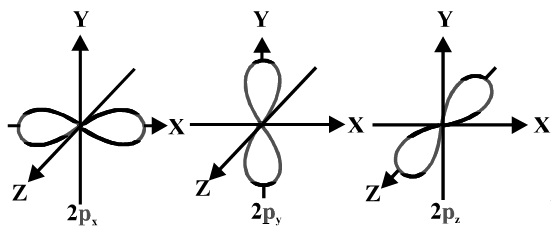
A #p# orbital consists of two lobes of electron density on either side of the nucleus.
We usually draw #p# orbitals as figure eights, but we should remember #p# orbitals are really much fatter than in our usual drawings.
Four of the five #d# orbitals consist of four lobes pointed towards the corners of a square, like a four-leaf clover. The fifth orbital looks like a #p# orbital with a doughnut around its middle.
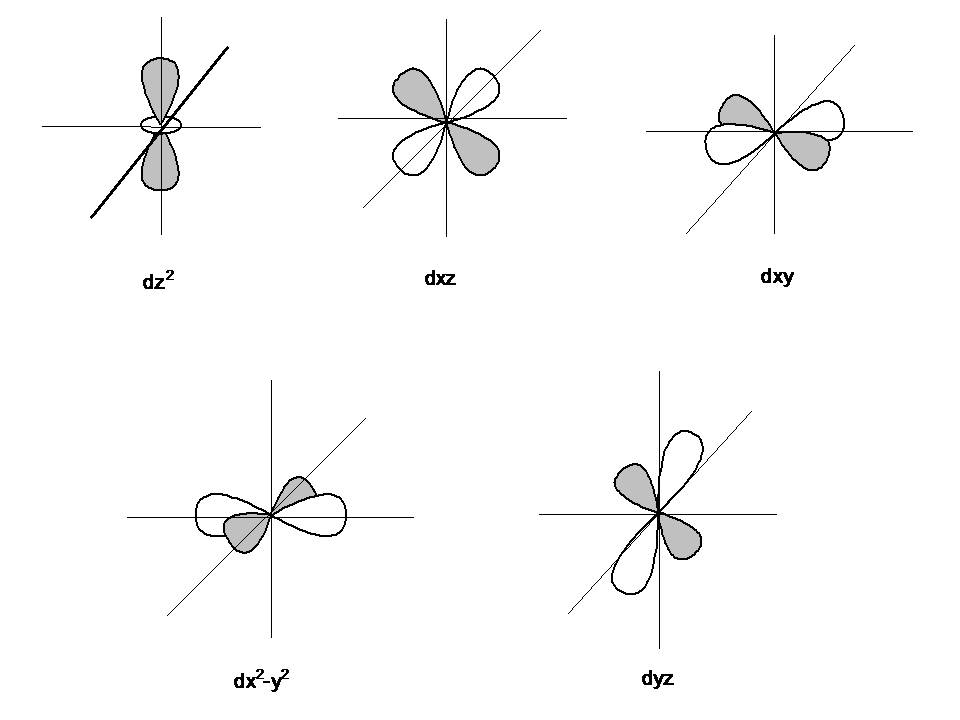
We usually draw #d# orbitals as skinny teardrops pointing in various directions, but we should remember that the lobes are really much fatter than in the drawing.
The seven #f# orbitals are even more complex shapes. The ones we see in diagrams are linear combinations of the #m_l# = -3 to +3 orbitals. Here is one common set of combinations.
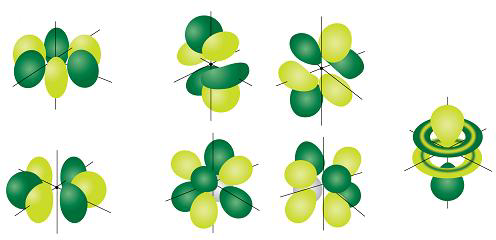
If you absolutely have to draw them, you can draw the shapes as below.

- One orbital looks like a #p# orbital with two doughnuts around its middle.
- Two orbitals have eight lobes pointing towards the corners of a cube.
- Four orbitals have six lobes oriented in various planes (easiest to draw).






