Question #e43ff
1 Answer
Jun 14, 2015
Rutherford concluded that an atom was mainly comprised of empty space and a small nucleus in the centre of the atom.
Explanation:
He arrived at this conclusion because most of the alpha particles went straight through the gold foil, whilst only some were deflected by the nucleus of the gold atoms in the foil.
Image from: 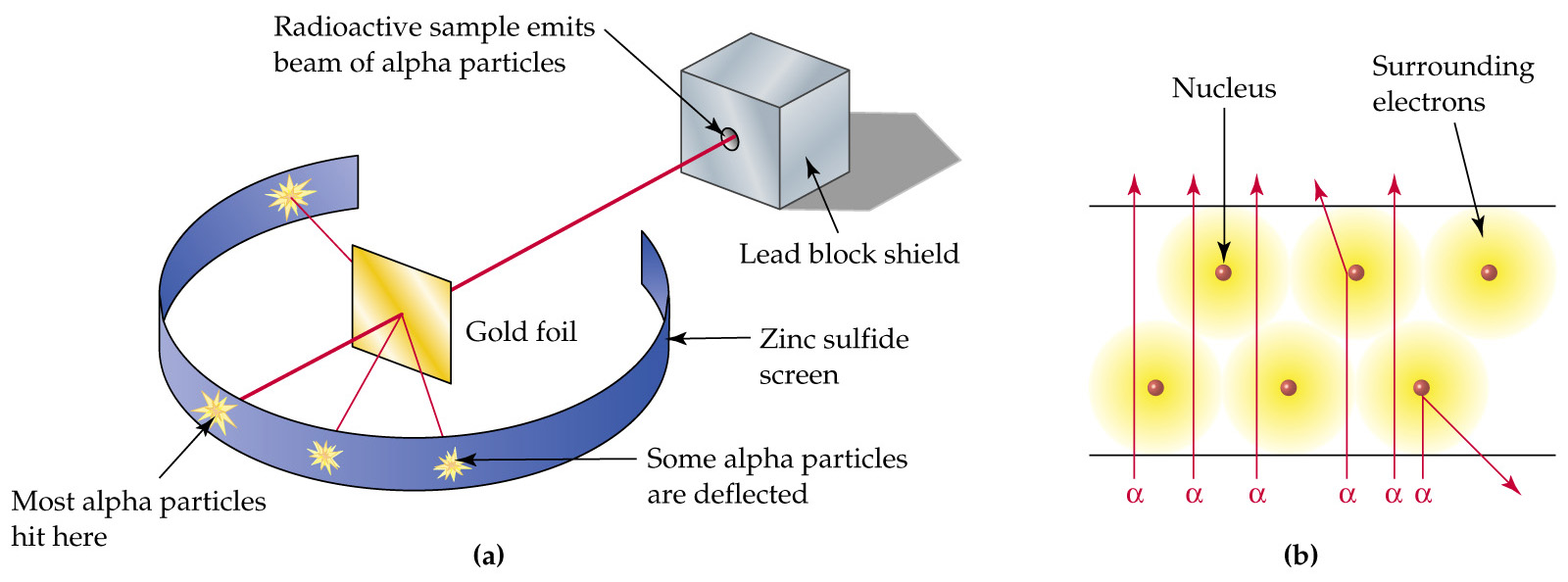
Hope I helped :)

