The reaction needs a protection step. Give full reaction, including protection and deprotection steps?
1 Answer
Here's what I get.
Explanation:
(a) Why do we need a protection step?
You are using potassium permanganate to oxidize an amine to a nitro group.
The problem is that the permanganate will also oxidize to aldehyde to a carboxylic acid.
You must first protect the aldehyde group, then do the oxidation, and then remove the protecting group to regenerate the aldehyde.
(b) The overall reaction
(i) Protection
A standard method of protecting an aldehyde group is to react it with
ethane-1,2-diol with p-toluenesulfonic acid in refluxing toluene.
The reaction is
The reaction is reversible, so you use a Dean-Stark apparatus to remove the water continuously as it forms.
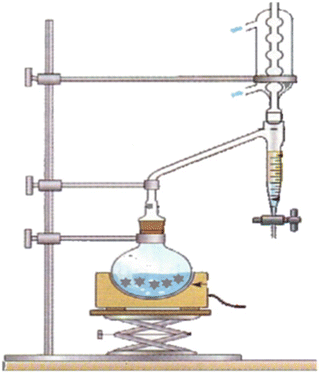
Dioxolanes are stable in basic solution, but they are sensitive to acid-catalyzed hydrolysis.
(b) Oxidation
Alkaline
(c) Deprotection
Treatment with aqueous acid hydrolyzes the dioxolane back to the aldehyde.

