Please help me with this question..... a)Polyethylene exists either as a linear (straight-chain) polymer or as a branched polymer. Which is the high density form? Explain.
b) Draw how the following monomer can be polymerised. What type of Polymerisation method is used?
![enter image source here]()
c) Draw how monomer H2C=CH2 can be polymerised. What type of polymerisation is method is used?
b) Draw how the following monomer can be polymerised. What type of Polymerisation method is used?
c) Draw how monomer H2C=CH2 can be polymerised. What type of polymerisation is method is used?
1 Answer
Here's what I find.
Explanation:
(a) Polyethylene
Linear polyethylene consists of chains of 7000 to 18 000 monomers.
There is always some branching, but it is possible to adjust conditions to control the degree of branching.
High-density polyethylene (HDPE) has about seven branches per thousand carbon atoms.
Low-density polyethylene (LDPE) has more than 60 branches per thousand carbons.
Here is a portion of an HDPE chain.
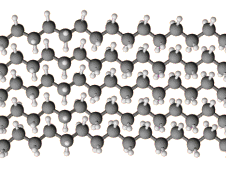
The linear chains pack tightly into a crystal structure, so the density of HDPE is high (
In contrast, the chains of LDPE have a more open structure.
The chains do not pack as well into a crystal structure.
The density of LDE is
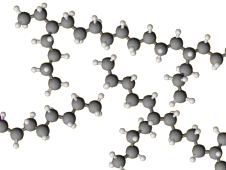
Thus, the straight-chain polymer is the high-density form.
(b) Polymerization of terephthalic acid
Terephthalic acid is a dibasic acid.
We could polymerize it by reaction with ethylene glycol to form an ester.
Then, the ends of each molecule could react with the corresponding ends of others to form a polyester.
This process of forming a polymer is a condensation reaction, in which two large molecules combine and split out a smaller one, like water.
(c) Polymerization of ethylene
One way to polymerize ethylene is to have the monomers add individually to the end of the growing chain.
This type of reaction is an **addition polymerization.

