Hydration via Hydroboration-Oxidation
Key Questions
-
Answer:
Hydroboration-oxidation is a method of making alcohols from alkenes.
Explanation:
It involves the addition of
#"BH"_3# to an alkene, followed by oxidation with alkaline hydrogen peroxide to form an alcohol.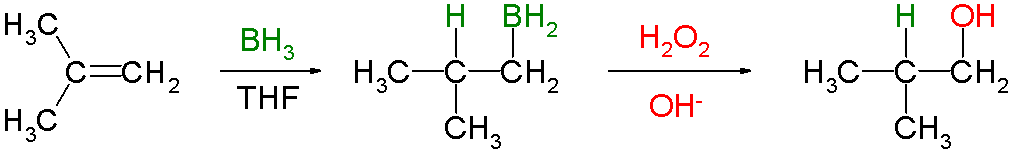
The reaction is a Markovnikov addition of
#"BH"_3# to the alkeneOn oxidation of the boron intermediate, the
#"OH"# group ends up on the less substituted carbon.This is opposite to the position of the
#"OH"# group in the acid-catalyzed Markovnikov addition of water to an alkene, so the reaction is often called the anti-Markovnikov addition of water to an alkene. -
Answer:
The borane-THF complex (BTHF) is used for hydroboration for reasons of safety and convenience.
Explanation:
The active ingredient is borane,
#"BH"_3# , but borane is a highly toxic gas.Borane exists naturally as the dimer
#"B"_2"H"_6# (diborane), but diborane mixes easily with air and forms explosive mixtures.Also, it ignites spontaneously in moist air at room temperature.
In a solution in THF, borane exists as a loose Lewis acid-base complex. This allows boron to have an octet and makes the reagent more stable.

The solution is commercially available in a 1 mol/L concentration in volumes from 25 to 800 mL.
It is much more convenient to work with the solution than with a gas. Even so, the solution must be stored at 2 to 8 °C, and it must have a stabilizer added.
Borane forms a more stable and more soluble Lewis acid-base complex with dimethyl sulfide:
#"H"_3stackrelcolor(blue)("-")("B")"-"stackrelcolor(blue)(+)("S")("CH"_3)_2# It is available in concentrations of 2, 5, and 10 mol/L and in volumes from 25 mL to 18 L.
That should make it a more convenient reagent than the BTHF complex.
There is only one problem: It has the highly disagreeable smell of rotten cabbage!
-
It's similar to for alkenes, but besides creating
#[B(OH)_4]^(-)# ,#H_2O# , and#OOH^(-)# (hydrogen peroxide's conjugate base), instead of getting the three#mols# of an alcohol, you have an enol. This enol can undergo keto-enol tautomerization.In this case it is in basic (base-ic) conditions, with
#M^(+)OH^(-)# available within the reaction vessel.An example of this is:
- First, the enol's
#R-OH# donates its proton to the base (#OH^(-)# from#M^(+)OH^(-)# ) to form an enolate. (#H_2O# forms, now) - Then, the enolate's oxygen moves its electrons down to form a
#pi# bond (double bond = 1#sigma# + 1#pi# ) and the#pi# bond down at the bottom donates its pi electrons to the resultant#H_2O# that just formed, grabbing a proton off and reforming the base (#OH^(-)# from#M^(+)OH^(-)# ).
You then form a ketone or aldehyde, depending on the location of the triple bond. Also, remember that hydroboration is anti-Markovnikov.
- First, the enol's