Radical Halogenation of Alkenes (Antimarkovnikov)
Key Questions
-
Answer:
From the mechanism I know, it helps in figuring out the products of electrophilic addition.
Explanation:
Electrophilic addition occurs when an alkene is added with a HX, where X is a halide.
So in a carbon chain, preferably propene, the double bond will first induce a +ve charge on to the hydrogen from HX. Hence X now has a -ve charge.
So the double bond breaks and the hydrogen and X halide bond to the carbon chain.
Markovnikov's rule helps here by explaining where the H+ and X- ion fix themselves and hence stating the major products of the reactions.During the formation of the intermediate, there is a carbocation. Depending on the stability of this carbocation, the position of the X- halide will vary. Hence resulting in either eg. 1-bromopropane or 2-bromopropane.
-
Anti-Markovnikov addition to a
#pi# bond requires the addition of the non-hydrogen group to the less substituted carbon.When a carbocation intermediate forms, it usually seeks to stabilize itself through rearrangements: which are accomplished through methyl or hydride shifts.
Hence, it will generally become more substituted, and Markovnikov addition will take place, as a result.
When we have a radical initiator, like
#HOOH# , we can ensure that the radical intermediate (that has had the halogen added to the#pi# bond, already, picture below) becomes the most stable which will undergo hydrogen abstraction with#HBr# .
If you want a detailed mechanism, ask!
-
An anti-Markovnikov halogenation is the free-radical addition of hydrogen bromide to an alkene.
In the Markovnikov addition of HBr to propene, the H adds to the C atom that already has more H atoms. The product is 2-bromopropane.
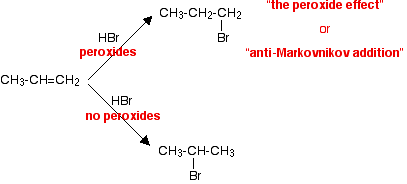
In the presence of peroxides, the H adds to the C atom that has fewer H atoms. This is called anti-Markovnikov addition. The product is 1-bromopropane.

The reason for anti-Markovnikov addition is that it is the Br atom that attacks the alkene. It attacks the C atom with the most H atoms, so the H adds to the C atom with the fewest H atoms.
Here is a video on the anti-Markovnikov addition of HBr to alkenes.